
- #ABBOTT SPINAL CORD STIMULATOR SOFTWARE#
- #ABBOTT SPINAL CORD STIMULATOR TRIAL#
- #ABBOTT SPINAL CORD STIMULATOR BLUETOOTH#
- #ABBOTT SPINAL CORD STIMULATOR SERIES#
The FDA data contains more than 500 reports of people with spinal-cord stimulators who died, but details are scant, making it difficult to determine if the deaths were related to the stimulator or implant surgery. Among the 4,000 types of devices tracked by the FDA, only metal hip replacements and insulin pumps have logged more injury reports.

Patients report that they have been shocked or burned or have suffered spinal-cord nerve damage ranging from muscle weakness to paraplegia, FDA data shows. Food and Drug Administration, with more than 80,000 incidents flagged since 2008.

They account for the third-highest number of medical device injury reports to the U.S. and as a treatment for an aging population in need of chronic pain relief.īut the stimulators-devices that use electrical currents to block pain signals before they reach the brain-are more dangerous than many patients know, an Associated Press investigation found. Companies and doctors aggressively push them as a safe antidote to the deadly opioid crisis in the U.S. "But look at me."įor years, medical device companies and doctors have touted spinal-cord stimulators as a panacea for millions of patients suffering from a wide range of pain disorders, making them one of the fastest-growing products in the $400 billion medical device industry. "I thought I would have a wonderful life," Taft said. Today, the 45-year-old Taft is virtually paralyzed, a prisoner in his own bed, barely able to get to the bathroom by himself. After an operation to repair it, he said, the device shocked him so many times that he couldn't sleep and even fell down a flight of stairs. Taft's stimulator failed soon after it was surgically implanted.
#ABBOTT SPINAL CORD STIMULATOR BLUETOOTH#
‡ indicates a third party trademark, which is property of its respective owner.īluetooth is a registered trademark of Bluetooth SIG, Inc.It wouldn't fix the nerve damage in his mangled right arm, Taft and his wife recalled the doctor saying, but a spinal-cord stimulator would cloak his pain, making him "good as new."
#ABBOTT SPINAL CORD STIMULATOR SOFTWARE#
It’s the only DBS system available to offer directional leads to potentially reduce side effects and Apple‡ iOS‡ software and patient controllers.įor people living with Parkinson’s, essential tremor and other movement disorders as well as those managing chronic pain, especially in their extremities, our Neuromodulation lineup is helping them live their best lives. Our Infinity TM Deep Brain Stimulation (DBS) system uses mild pulses of electricity to the brain, alleviating symptoms of Parkinson’s disease and essential tremor.
#ABBOTT SPINAL CORD STIMULATOR TRIAL#
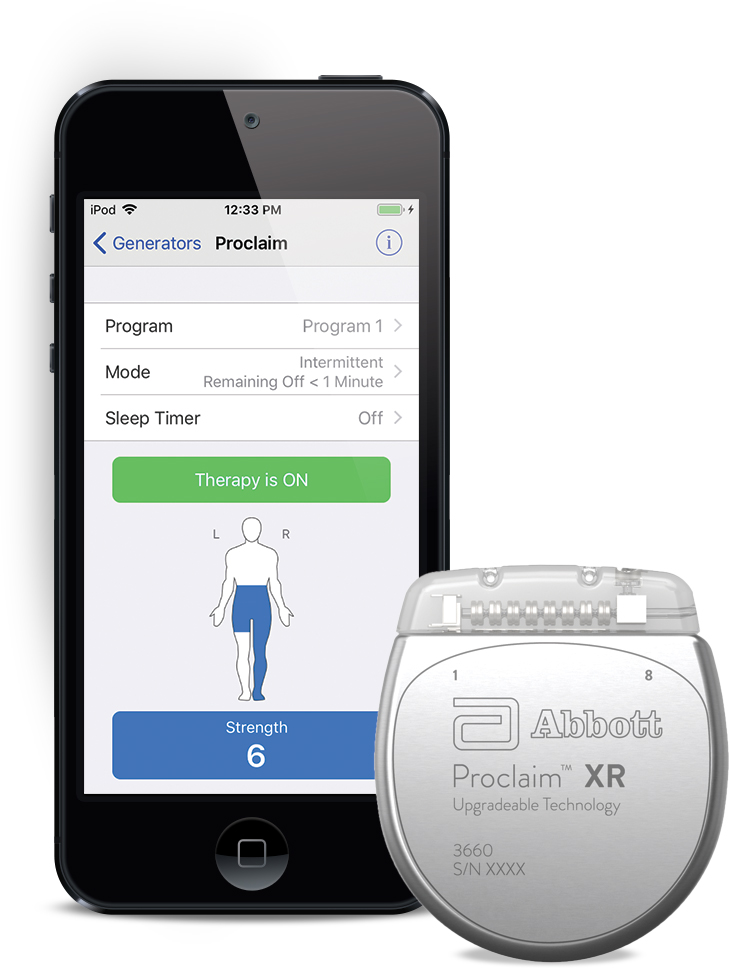
Our Invisible Trial System TM is a discreet, app-based and wireless system that leverages Apple‡ iPod touch‡ mobile digital devices to help patients evaluate SCS therapy. The Proclaim TM SCS System is also the first upgradeable and recharge-free spinal cord stimulation system capable of delivering both tonic stimulation and Abbott’s proprietary BurstDR stimulation waveform, a superior therapy designed to more closely mimic how pain signals travel to the brain. The Proclaim platform’s Bluetooth ® wireless technology and iOS‡ software offers patients an intuitive therapy experience. The Proclaim TM DRG Neurostimulation System is the first and only neurostimulation device approved only for CRPS.
#ABBOTT SPINAL CORD STIMULATOR SERIES#
Our Proclaim series includes devices designed to deliver spinal cord stimulation (SCS) for the treatment of chronic pain, and dorsal root ganglion (DRG) stimulation for patients seeking relief from causalgia - nerve pain following surgery or injury - and complex regional pain syndrome (CRPS). We offer specialized devices for people suffering from chronic pain and movement disorders through solutions that deliver stimulation to the spinal cord, dorsal root ganglion and the brain. For people living with chronic pain and movement disorders, our portfolio of therapies help them move and feel better, allowing chronic pain patients to reduce or stabilize the long-term use of opioids and get back to living their lives, while helping patients with movement disorders combat the symptoms of their condition.


 0 kommentar(er)
0 kommentar(er)
